Introduction
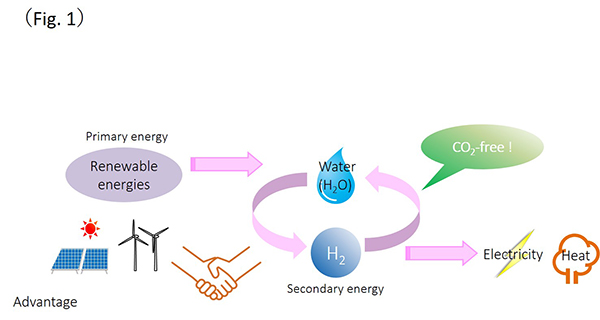
Global warming is now in a more serious stage and we are facing an environment crisis. Our urgent issue is how to reduce carbon dioxide (CO2) quickly from our planet. To make a decarbonized society possible, we must be able to install and utilize renewable energies in greater quantity. However, renewable energies are easily influenced by the weather, and they cannot obtain high density. In addition, the electricity from renewable energies is hard to storage in a large scale for a long term. In order to overcome these weak points about renewable energies, hydrogen can be used for its conversion and storage as well as transformation. Moreover, hydrogen itself can be utilized for energy resources (Fig. 1).
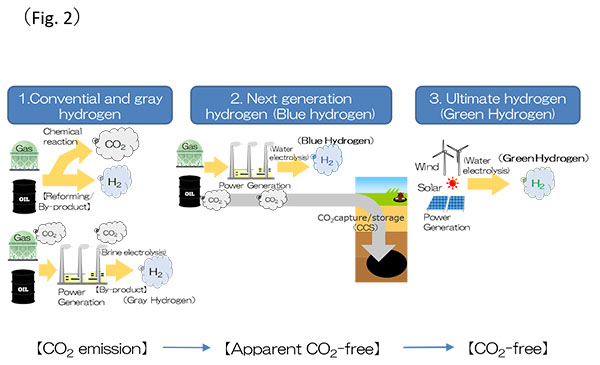
The conventional method to obtain hydrogen is through the chemical reaction of gas and oil, but this causes a certain amount of CO2 emission. Another method is through the brine electrolysis: the electricity used for the electrolysis is produced from gas and oil, and hydrogen as a by-product. However, this also emits some CO2. Hydrogen obtained through such conventional methods is called “Gray hydrogen”. On the other hand, a new method to produce hydrogen is through the water electrolysis using the technique of carbon capture and storage (CCS). It has apparently “zero” emission of CO2 and such hydrogen is called “Blue hydrogen”. Furthermore, it is expected to produce hydrogen in the future by the water electrolysis using with electricity from renewable energies. It has perfectly zero emission of CO2 throughout the production process. This truly CO2-free hydrogen is called “Green hydrogen”* (Fig. 2).
* K. Ota, A. Ishihara, K. Matsuzawa, and S. Mitsushima, Electrochemistry, 78, 970 (2010).
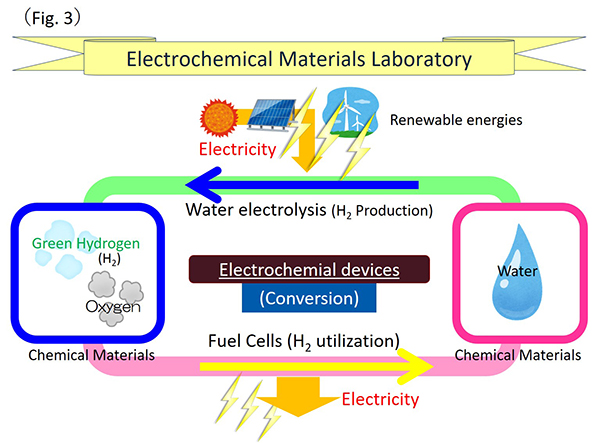
Electrochemical materials laboratory (Matsuzawa group) was established in April 2018 at the Division of Materials Science and Chemical Engineering, Graduate School of Engineering in YNU. The mission of our laboratory is to research and develop innovative electrocatalysts in order to contribute not only for Sustainable Development Goals (SDGs) which is the proposal from the United Nations but also to a further sustainable society in the future. From academic aspect, our laboratory is to study for the electrode reaction related to electrochemical reaction occurring at the interface. Our study is to contribute to the technique for energy storage and energy utilization by the conversion between chemical and electrical energies. From the engineering aspect, our laboratory is to developed materials for electrochemical devices that will be required for the “Green hydrogen” society, specially to improve the high performance and durability for fuel cell as a hydrogen utilization device as well as electrode materials for the water electrolysis as a hydrogen production device. Moreover, we focus on these devices to apply for the utilization and production for the “Green hydrogen”, and to elucidate electrochemically the reaction mechanism of oxygen electrode reaction (Fig. 3).
Our laboratory supervised by Koichi Matsuzawa would like to establish international collaboration for the subject “Green Hydrogen” and always welcomes those who are interested in the subject including the development of fuel cell and water electrolysis. Please feel free to contact us by e-mail if you are interested or have any questions:

